Tremfya (guselkumab) / J&JXi'an Janssen announces the first targeted interleukin 23 inhibitor Tenoya (gusakiyu mab) [Google translation] (Sina Corp) - Apr 21, 2020 - "Johnson & Johnson's subsidiary in China, Xi'an Janssen Pharmaceutical Co., Ltd. recently announced that its Tenova (Gusakiyu monoclonal antibody injection, English trade name: TREMFYA, Guselkumab) has been launched in China and is used for systemic treatment....The China Red Cross Foundation launched the 'Special Rebirth' assistance project for patients with moderate to severe plaque psoriasis, providing Tenova (Gusai) to patients with moderate to severe plaque psoriasis with low income and low security Chiyuzumab injection) drug assistance."
bimekizumab (UCB4940) / UCBBE COMPLETE: A Study to Evaluate the Efficacy and Safety of Bimekizumab in the Treatment of Subjects With Active Psoriatic Arthritis (clinicaltrials.gov) - Apr 20, 2020 - P3; N=390; Suspended; Sponsor: UCB Biopharma S.P.R.L.; Recruiting --> Suspended
bimekizumab (UCB4940) / UCB; Humira (adalimumab) / Eisai, AbbVieBE OPTIMAL: A Study to Test the Efficacy and Safety of Bimekizumab in the Treatment of Subjects With Active Psoriatic Arthritis (clinicaltrials.gov) - Apr 20, 2020 - P3; N=840; Suspended; Sponsor: UCB Biopharma S.P.R.L.; Recruiting --> Suspended
Skyrizi (risankizumab) / AbbVie, Boehringer IngelheimAbbVie announces the availability in Spain of risankizumab [Google translation] (ConSalud.es) - Apr 21, 2020 - "The biopharmaceutical company AbbVie announces the availability in Spain from April 1 of SKYRIZI (risankizumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic treatment....This approval is based on the robust clinical development that includes the pivotal phase III studies: UltIMMa-1, UltIMMa-2, IMMvent and IMMhance, which have evaluated the long-term safety and efficacy of this new treatment in more than 2,100 patients with moderate to severe plaque psoriasis." "
Taltz (ixekizumab) / Eli LillyLilly reports strong first-quarter financial results, adjusts EPS guidance (Eli Lilly Press Release) - Apr 23, 2020 - P4, N=1,028; IXORA-R (NCT03573323); Sponsor: Eli Lilly and Company; "The company completed a Phase 4 study of Taltz in patients with moderate to severe psoriasis. Taltz demonstrated non-inferiority to guselkumab on the final secondary endpoint at week 24. As previously disclosed, Taltz achieved superiority compared to guselkumab on all primary and key secondary endpoints at week 12."
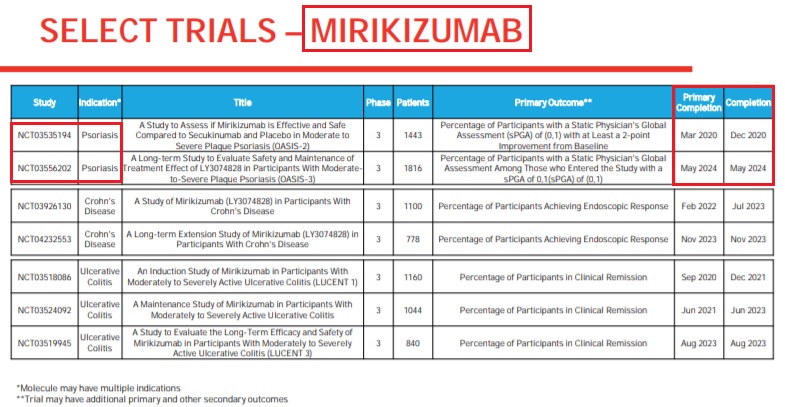
mirikizumab (LY3074828) / Eli LillyEli Lilly and Company (LLY) CEO Dave Ricks on Q1 2020 results - Earnings call transcript (SeekingAlpha) - Apr 23, 2020 - P3, N=530; OASIS-1 (NCT03482011); P3, N=1,443; OASIS-2 (NCT03535194); Sponsor: Eli Lilly and Company; "We also announced the first of two Phase 3 trials studying mirikizumab in psoriasis met its primary endpoints...We announced that mirikizumab met all the co-primary and key secondary endpoints in the first OASIS-1 trial, which is the placebo-controlled 52 weeks trial in psoriasis."
Skyrizi (risankizumab) / AbbVie, Boehringer IngelheimA Study to Assess the Safety and Efficacy of Risankizumab for Maintenance in Moderate to Severe Plaque Type Psoriasis ( LIMMITLESS ) (clinicaltrials.gov) - Apr 24, 2020 - P3; N=2171; Active, not recruiting; Sponsor: AbbVie; Enrolling by invitation --> Active, not recruiting
mirikizumab (LY3074828) / Eli LillyMirikizumab: Primary completion of P3 OASIS-2 trial (NCT03535194) in moderate-to-severe plaque psoriasis in Mar 2020 (Eli Lilly) - Apr 23, 2020 - Q1 2020 Results: Completion of P3 OASIS-2 trial in moderate-to-severe plaque psoriasis in Dec 2020; Primary completion and completion of P3 OASIS-3 trial (NCT03556202) in moderate-to-severe plaque psoriasis in May 2024
Simponi (golimumab) / Merck (MSD), Mitsubishi Tanabe, J&JJanssen announces submission of two applications to U.S. FDA seeking approval of Simponi Aria (golimumab) for the treatment of polyarticular juvenile idiopathic arthritis and juvenile psoriatic arthritis (PRNewswire) - Apr 24, 2020 - "The Janssen Pharmaceutical Companies of Johnson & Johnson today announced the submission of two supplemental Biologics License Applications (sBLA) to the U.S. Food and Drug Administration (FDA) seeking approval of SIMPONI ARIA® (golimumab) for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) and juvenile psoriatic arthritis (jPsA), in patients two years of age and older in combination with methotrexate....The submissions are based on results from the GO-VIVA Phase 3 clinical trial, which...assess the pharmacokinetics, safety and efficacy of SIMPONI ARIA in children with pJIA ages two to 17 years who had active arthritis in five or more joints..."
Stelara (ustekinumab) / J&JStelara: Patent expiry in US in 2023 (J&J) - Apr 26, 2020 - Annual Meeting of Shareholders