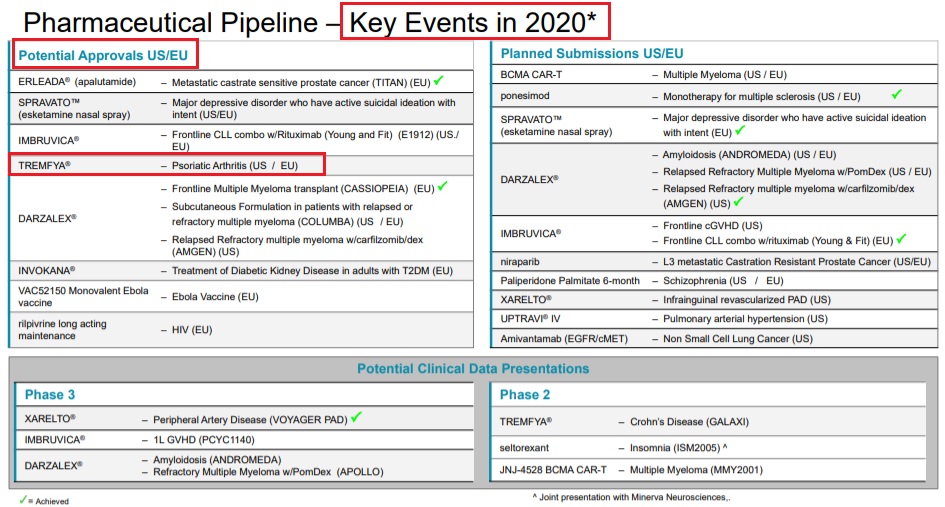
Tremfya (guselkumab) / J&JTremfya: Regulatory approval in US/EU for psoriatic arthritis in 2020 (J&J) - Apr 14, 2020 - Key 2020 Events
Otezla (apremilast) / AmgenScalp psoriasis study data added to Otezla labeling (eMPR) - Apr 14, 2020 - "The labeling for Otezla (apremilast; Amgen) has been updated to include safety and efficacy information related to the treatment of patients with moderate to severe plaque psoriasis of the scalp....Results showed that a larger proportion of patients treated with Otezla had ScPGA response compared with placebo (43.3% vs 13.7%; treatment difference: 29.6%; 95% CI, 19.5-39.7). In addition, a greater percentage of Otezla-treated patients had Whole Body Itch NRS response and Scalp Itch NRS response (45.5% and 47.1%, respectively) vs placebo-treated patients (22.5% and 21.1%, respectively)."
Cosentyx (secukinumab) / NovartisEXCEED 1: Efficacy of Secukinumab Compared to Adalimumab in Patients With Psoriatic Arthritis (clinicaltrials.gov) - Apr 14, 2020 - P3; N=854; Completed; Sponsor: Novartis Pharmaceuticals; Active, not recruiting --> Completed; Trial primary completion date: Sep 2019 --> Dec 2019
Amjevita (adalimumab biosimilar) / Amgen, Daiichi SankyoAmgevita approved in Colombia (GaBI) - Apr 17, 2020 - "Amgen’s Amgevita (ABP 501) is the first producto bioterapéutico similar to be approved in Colombia for the treatment of complex diseases such as rheumatoid arthritis, Crohn’s disease and psoriasis."
Cosentyx (secukinumab) / NovartisNovartis: Recent selloff has presented a buying opportunity (SeekingAlpha) - Apr 13, 2020 - "In 2019, Cosentyx reported revenues of $3.6 billion, a YoY jump of 28%. This is mainly driven by robust uptake in dermatology and rheumatology indications. Novartis now expects Cosentyx's peak sales to exceed $5.0 billion."
Otezla (apremilast) / AmgenA Long-term Extension Study of Apremilast (CC-10004) in Pediatric Subjects From 6 Through 17 Years of Age With Moderate to Severe Plaque Psoriasis (clinicaltrials.gov) - Apr 17, 2020 - P3b; N=140; Recruiting; Sponsor: Amgen; Not yet recruiting --> Recruiting
Ilumya (tildrakizumab-asmn) / Sun Pharma, AlmirallEfficacy and Safety of Tildrakizumab Compared to Placebo in Anti-TNF naïve Subjects With Active Psoriatic Arthritis II (INSPIRE 2) (clinicaltrials.gov) - Apr 13, 2020 - P3; N=292; Not yet recruiting; Sponsor: Sun Pharma Global FZE; N=50 --> 292
Ilumya (tildrakizumab-asmn) / Sun Pharma, AlmirallEfficacy and Safety of Tildrakizumab Compared to Placebo in Subjects With Active Psoriatic Arthritis I (INSPIRE 1) (clinicaltrials.gov) - Apr 13, 2020 - P3; N=472; Not yet recruiting; Sponsor: Sun Pharma Global FZE; N=200 --> 472
Cimzia (certolizumab pegol) / Astellas, UCB, Eli LillyCIMcare: A Study to Evaluate the Efficacy, Safety, and Drug Concentration of Certolizumab Pegol (CZP) in Children and Adolescent Study Participants With Moderate to Severe Chronic Plaque Psoriasis (PSO) (clinicaltrials.gov) - Apr 17, 2020 - P3; N=150; Suspended; Sponsor: UCB Biopharma S.P.R.L.; Recruiting --> Suspended