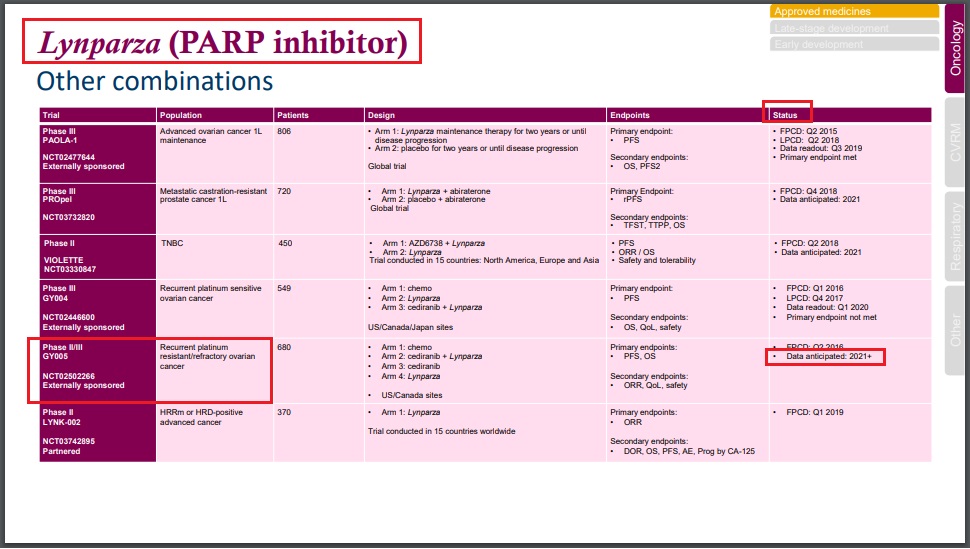
Lynparza (olaparib) / Merck (MSD), AstraZeneca; Recentin (cediranib) / AstraZenecaLynparza + Recentin: Data from P2/3 trial (NCT02502266) for recurrent platinum resistant/refractory ovarian cancer in 2021 or later (AstraZeneca) - Apr 29, 2020 - Q1 2020 Results
llllllllll Zejula (niraparib) / GSK, ZAI Lab, J&J, TakedaFDA approves Zejula (niraparib) as the only once-daily PARP inhibitor in first-line monotherapy maintenance treatment for women with platinum-responsive advanced ovarian cancer regardless of biomarker status (PRNewswire) - Apr 29, 2020 - "GlaxoSmithKline plc...announced the US Food and Drug Administration (FDA) approved the company's supplemental New Drug Application (sNDA) for Zejula (niraparib), an oral, once-daily poly (ADP-ribose) polymerase (PARP) inhibitor, as a monotherapy maintenance treatment for women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to first-line platinum-based chemotherapy, regardless of biomarker status. Until now, only 20% of women with ovarian cancer, those with a BRCA mutation (BRCAm), were eligible to be treated with a PARP inhibitor as monotherapy in the first-line maintenance setting....This new indication is supported by data from the phase III PRIMA study (ENGOT-OV26/GOG-3012)..."
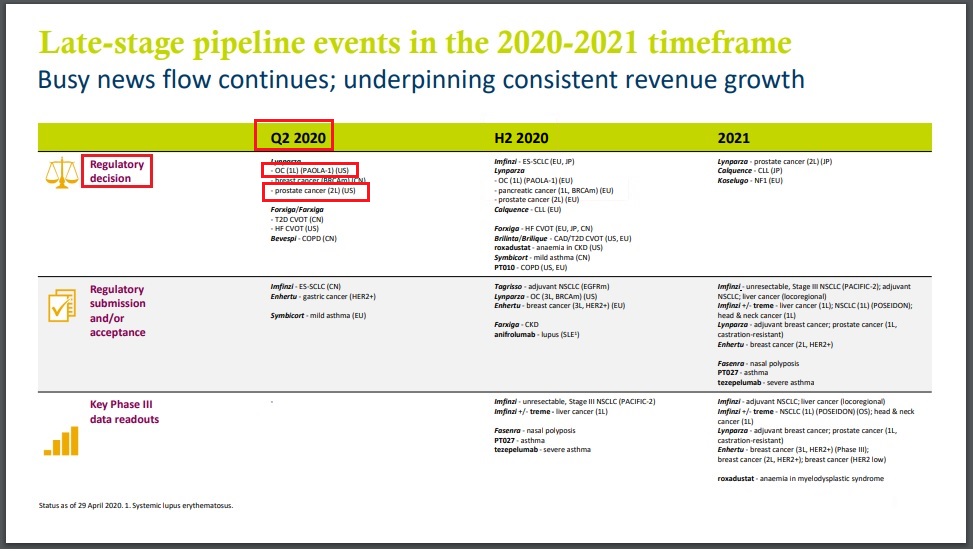
Lynparza (olaparib) / Merck (MSD), AstraZenecaLynparza: Regulatory decision in US (based on PAOLA trial) for ovarian cancer in Q2 2020 (AstraZeneca) - Apr 29, 2020 - Q1 2020 Results: Regulatory decision in US for 2L prostate cancer in Q2 2020
Zejula (niraparib) / GSK, ZAI Lab, J&J, Takeda; dostarlimab (TSR-042) / GSKZejula + dostarlimab: Regulatory submission for 2L+ ovarian cancer (based on MOONSTONE trial) in H2 2021 (GSK) - Apr 30, 2020 - Q1 2020 Results

NEO-PTC-01 / Neon TherapeuticsNeon Therapeutics announces acceptance of European Clinical Trial Authorization application for NEO-PTC-01 (GlobeNewswire) - Apr 30, 2020 - “Neon Therapeutics, Inc…announced the acceptance of its Clinical Trial Authorization (CTA) from the Dutch Health Authority (DHA) for its personal neoantigen-targeted T cell therapy candidate, NEO-PTC-01. Neon’s initial Phase 1 clinical trial of NEO-PTC-01 will be in patients with metastatic melanoma who are not responsive to checkpoint inhibitors. The Phase 1 dose-finding clinical trial in metastatic melanoma will be conducted in collaboration with the Netherlands Cancer Institute and is expected to begin in the third quarter of 2020… Neon is also planning for a second indication for NEO-PTC-01 in metastatic ovarian cancer, as well as for the potential to both expand to other solid tumor types and pursue clinical development in the United States.”
mirvetuximab soravtansine (IMGN 853) / ImmunoGen; IMGN151 / ImmunoGenImmunoGen reports recent progress and first quarter 2020 financial results (Businesswire) - May 1, 2020 - “ANTICIPATED UPCOMING EVENTS: Present updated data from the FORWARD II platinum-sensitive triplet cohort evaluating mirvetuximab in combination with carboplatin and bevacizumab in the fall; Continue enrollment in IMGN632 monotherapy and combination cohorts; File IND for IMGC936 at the end of Q2; Transition IMGN151 into pre-clinical development.”
Lynparza (olaparib) / Merck (MSD), AstraZenecaCost-effectiveness of upfront maintenance therapies for advanced ovarian cancer (Journal of Clinical Pathways) - Apr 29, 2020 - "...researchers found the estimated cost of olaparib in metastatic BRCA patients was $16,178 per month for 2 years of maintenance, based on the data from SOLO-1. With an estimated 48-month improvement in PFS, the ICER of olaparib would be $96,000 per progression-free life-year saved. Finally, assuming hypothetical results of PAOLA-1 at a 36-, 48, and 60-month improvements in PFS, the ICER of bevacizumab plus olaparib would be $347,428, $270,222, and $221,090 per progression-free life-year saved, respectively."
mirvetuximab soravtansine (IMGN 853) / ImmunoGen; IMGN151 / ImmunoGenImmunoGen reports recent progress and first quarter 2020 financial results (Businesswire) - May 1, 2020 - “ANTICIPATED UPCOMING EVENTS: Continue patient enrollment in pivotal SORAYA and confirmatory MIRASOL trials; Support initiation of an additional platinum-sensitive investigator sponsored trial evaluating mirvetuximab in combination with carboplatin in over 100 patients; Present initial data from the FORWARD II platinum-agnostic doublet cohort evaluating mirvetuximab in combination with bevacizumab in an oral presentation at the virtual American Society of Clinical Oncology (ASCO) Annual Meeting in May; Present pre-clinical data evaluating our next generation anti-folate receptor alpha (FRα) ADC, IMGN151, in ovarian cancer and other tumor types in a poster at the virtual American Association for Cancer Research (AACR) Annual Meeting in June.”
Lynparza (olaparib) / Merck (MSD), AstraZenecaSOLO-2: Olaparib Treatment in BRCA Mutated Ovarian Cancer Patients After Complete or Partial Response to Platinum Chemotherapy (clinicaltrials.gov) - Apr 28, 2020 - P3; N=327; Active, not recruiting; Sponsor: AstraZeneca; Trial completion date: Apr 2020 --> Feb 2022
ovapuldencel-T (AVOVA-1) / Caladrius, AiVita BiomedicalAIVITA Biomedical presents updates from ongoing ovarian cancer and glioblastoma clinical studies at 2020 AACR Virtual Annual Meeting (Businesswire) - Apr 27, 2020 - P2, N=99; NCT02033616; Sponsor: Aivita Biomedical, Inc; "The presentation from Lisa Abaid, MD, principal investigator of the Phase 2 clinical trial of AVOVA-1 in advanced ovarian cancer, showed the trial is progressing as planned and the treatment has been well-tolerated. Enrollment for the study continues for both tumor collection and randomization."