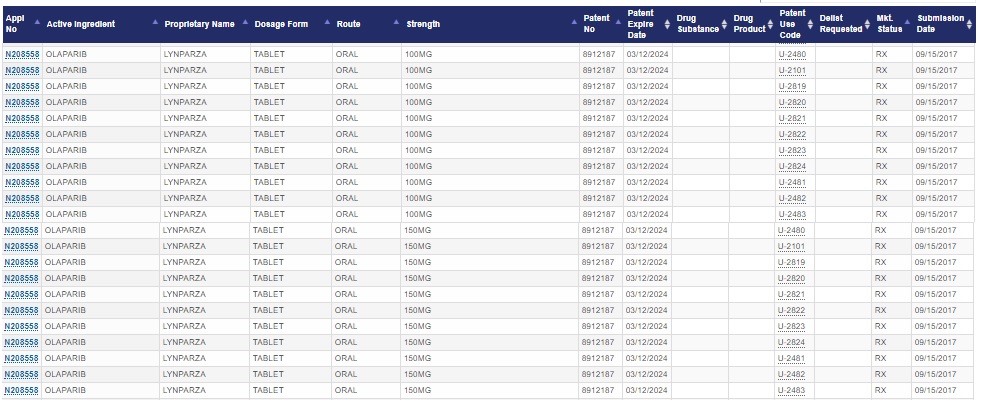
Lynparza (olaparib) / Merck (MSD), AstraZenecaLynparza: “Median OS improved by 12.9 months with maintenance Lynparza over placebo (p-value: 0.0537)”; Ovarian cancer (AstraZeneca) - Jun 2, 2020 - ASCO 2020
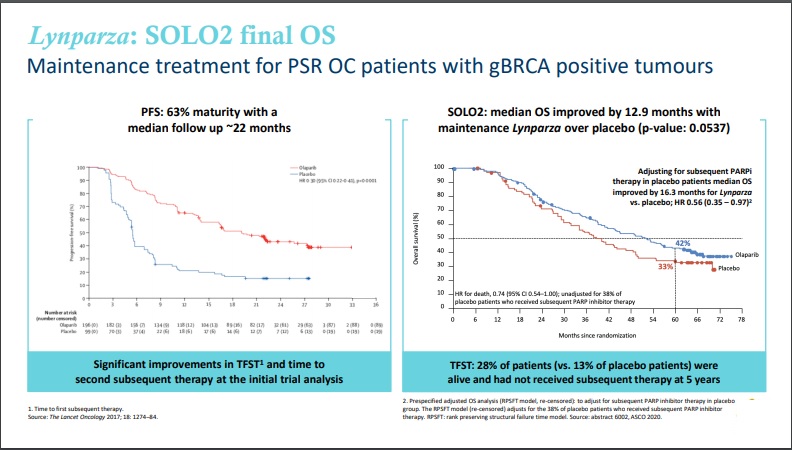
Rubraca (rucaparib) / ClovisRubraca: Data from monotherapy portion of P3 ATHENA trial (NCT03522246) for 1L ovarian cancer in H2 2021 (Clovis Oncology) - Jun 5, 2020 - Corporate Presentation
olvimulogene nanivacirepvec (GL-ONC1) / GeneluxGenelux announces formation of clinical advisory board on gynecologic cancers (PRNewswire) - Jun 4, 2020 - "Genelux Corporation...announced that it has formed a Clinical Advisory Board (CAB) on gynecologic cancers that will guide clinical development of its lead clinical-stage candidate, Olvi-Vec (olvimulogene nanivacirepvec). Genelux has completed enrollment of VIRO-15, a multi-center, open-label, Phase 2 study (NCT02759588) testing Olvi-Vec in platinum-resistant/refractory ovarian cancer and is in the follow up phase of the trial....'we look forward to their insight and expertise in helping the company advance our promising Olvi-Vec program into Phase 3....Abbreviated biographies of Genelux's inaugural CAB members are as follows...Robert L. Coleman, MD...Thomas J. Herzog, MD..."
Zejula (niraparib) / GSK, ZAI Lab, J&J, TakedaClinical Trial Evaluating the Efficacy and Safety of ZL-2306 (Niraparib) in Ovarian Cancer Patient (clinicaltrials.gov) - Jun 2, 2020 - P3; N=265; Recruiting; Sponsor: Zai Lab (Shanghai) Co., Ltd.; Trial completion date: Jun 2020 --> Apr 2021; Trial primary completion date: Jun 2020 --> Feb 2020
ofranergene obadenovec (VB-111) / NanoCarrier, VBL TherapeuticsVBL presents positive interim data from the OVAL phase 3 pivotal study in ovarian cancer at the ASCO20 Annual Meeting, showing 58% or higher objective response rate (GlobeNewswire) - Jun 1, 2020 - P3, N=400; NCT03398655; Sponsor: VBL Therapeutics; "OVAL independent Data Safety Monitoring Committee (DSMC) reviewed un-blinded data and determined that the study has met the interim pre-specified criterion of an absolute percentage advantage of 10% or higher in CA-125 response in the VB-111 treated arm compared to control. The DSMC recommended that the study proceed without modification. Overall CA-125 response rate in the first 60 randomized evaluable patients is 53%. Assuming a balanced randomization, the response rate in the treatment arm (VB-111 in addition to weekly paclitaxel) is 58% or higher. In patients with post-treatment fever, the CA-125 response is 69%. Fever is frequently observed after VB-111 treatment. The next interim analysis in the OVAL study is expected in 3Q 2020."
Lynparza (olaparib) / Merck (MSD), AstraZenecaLynparza: Newly added patent in Orange Book (Orange Book) - Jun 5, 2020 - Expiry on March 12, 2024