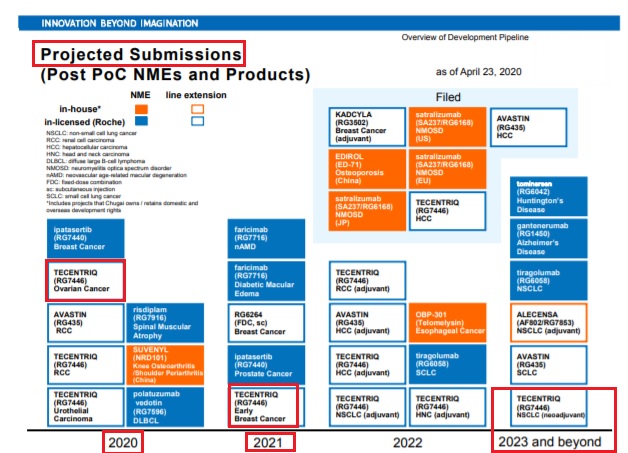
Tecentriq (atezolizumab) / RocheTecentriq: Regulatory submission in Japan for ovarian cancer in 2020 (Chugai) - Apr 23, 2020 - Q1 FY 2020 Results: Regulatory submission in Japan for early breast cancer in 2021; Regulatory submission in Japan for NSCLC (neoadjuvant) in 2023 or later
dostarlimab (TSR-042) / GSK; Zejula (niraparib) / GSK, ZAI Lab, J&J, TakedaA Phase 3 Comparison of Platinum-based Therapy With TSR-042 and Niraparib Versus Standard of Care (SOC) Platinum-based Therapy as First-line Treatment of Stage III or IV Nonmucinous Epithelial Ovarian Cancer (clinicaltrials.gov) - Apr 22, 2020 - P3; N=1228; Recruiting; Sponsor: Tesaro, Inc.; N=912 --> 1228; Trial completion date: Jul 2023 --> Jan 2023; Trial primary completion date: Nov 2021 --> Jul 2021
Keytruda (pembrolizumab) / Merck (MSD); LY 3475070 / Eli LillyA Study of the CD73 Inhibitor LY3475070 Alone or in Combination With Pembrolizumab in Participants With Advanced Cancer (clinicaltrials.gov) - Apr 22, 2020 - P1; N=120; Suspended; Sponsor: Eli Lilly and Company; Recruiting --> Suspended