etrasimod (APD334) / ArenaEtrasimod: Data from P2b CULTIVATE trial (NCT04173273) for Crohn’s disease in mid-2021 (Arena) - Jun 18, 2020 - Corporate Presentation: Data from P3 trial for Crohn’s disease in H2 2022
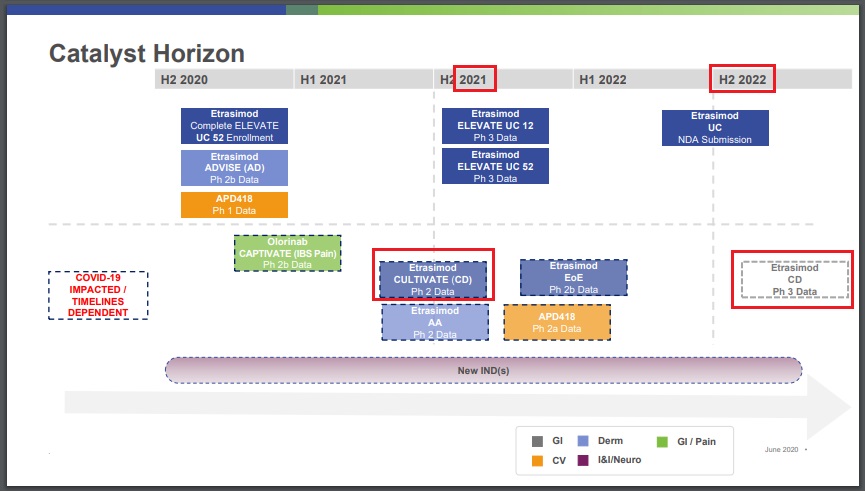
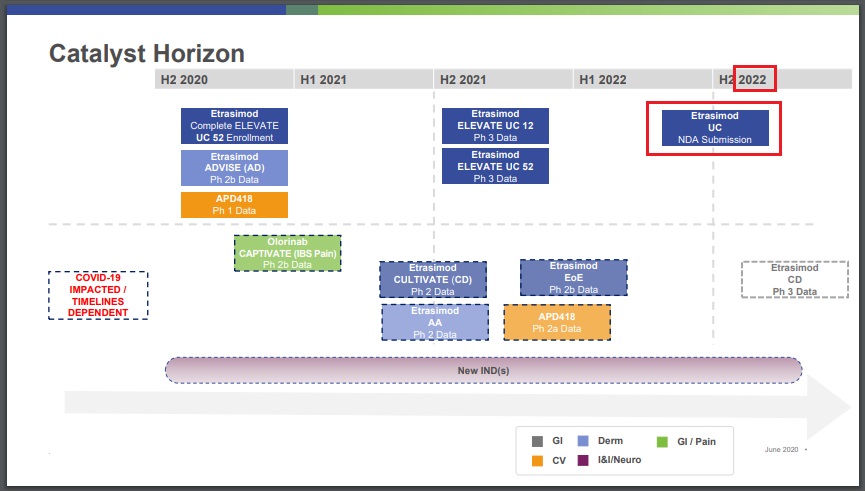
etrasimod (APD334) / ArenaEtrasimod: NDA submission for ulcerative colitis in mid-2022 (Arena) - Jun 18, 2020 - Corporate Presentation
Stelara (ustekinumab) / J&JFinal NICE green light for Janssen's Stelara in UC (PharmaTimes) - Jun 17, 2020 - "The National Institute for Health and Care Excellence (NICE) has now issued a final green light for NHS use of Janssen's Stelara (ustekinumab) to treat ulcerative colitis. The decision permits NHS doctors to prescribe the drug to treat moderately to severely active ulcerative colitis (UC) in adults when conventional therapy or a biological agent cannot be tolerated, or the disease has responded inadequately or lost response to treatment, only if: a tumour necrosis factor (TNF)-alpha inhibitor has failed, cannot be tolerated, or is unsuitable....According to NICE, clinical trial evidence shows that Stelara is more effective than placebo for treating moderately to severely active ulcerative colitis....However, its endorsement is contingent upon Janssen providing Stelara at the same price or lower than that agreed..."
Skyrizi (risankizumab) / AbbVie, Boehringer IngelheimA Multicenter, Randomized, Double-Blind, Placebo Controlled Induction Study to Evaluate the Efficacy and Safety of Risankizumab in Subjects With Moderately to Severely Active Ulcerative Colitis Who Have Failed Prior Biologic Therapy (clinicaltrials.gov) - Jun 17, 2020 - P2/3; N=720; Recruiting; Sponsor: AbbVie; N=1536 --> 720; Trial primary completion date: Sep 2020 --> Mar 2022
Xeljanz (tofacitinib) / Pfizer, Marche Polytechnic UniversityANMAT approved the use of a medicine to treat ulcerative colitis [Google translation] (El Litoral) - Jun 17, 2020 - "The National Administration of Medicines, Foods and Medical Technology (ANMAT) approved in Argentina the use of an oral medication for the treatment of moderate to severe ulcerative colitis in adults. Approval was obtained from the results of three phase III clinical trials that integrate a global clinical development program called OCTAVE and that included more than 1,700 patients. This is a new indication for a medicine containing tofacitinib for the treatment of ulcerative colitis..."
Zeposia (ozanimod) / BMSSafety and Efficacy Trial of RPC1063 for Moderate to Severe Ulcerative Colitis (clinicaltrials.gov) - Jun 16, 2020 - P3; N=1012; Active, not recruiting; Sponsor: Celgene; Recruiting --> Active, not recruiting; Trial completion date: Mar 2021 --> Jun 2020; Trial primary completion date: Jun 2020 --> Mar 2020
To Evaluate the Efficacy and Safety of FE 999315 in Japanese Subjects With Mild to Moderate Active Ulcerative Colitis (clinicaltrials.gov) - Jun 17, 2020 - P3; N=274; Completed; Sponsor: Ferring Pharmaceuticals; Active, not recruiting --> Completed
ALPN-101 / AbbVieAlpine Immune Sciences and AbbVie announce option and license agreement for the development and commercialization of ALPN-101 (Businesswire) - Jun 18, 2020 - "Alpine Immune Sciences...and AbbVie...announced an exclusive worldwide option and license agreement for ALPN-101, a first-in-class dual CD28/ICOS costimulation antagonist....Under the terms of the agreement, Alpine will receive an upfront payment of $60 million, and will also be eligible to receive up to an aggregate of $805 million for exercise of the option and success-based development, regulatory and commercial milestones. In addition, Alpine is eligible to receive tiered royalties on net sales of ALPN-101. In exchange, AbbVie will receive an option to an exclusive license for ALPN-101. During the option period, Alpine will conduct a phase 2 study in systemic lupus erythematosus."