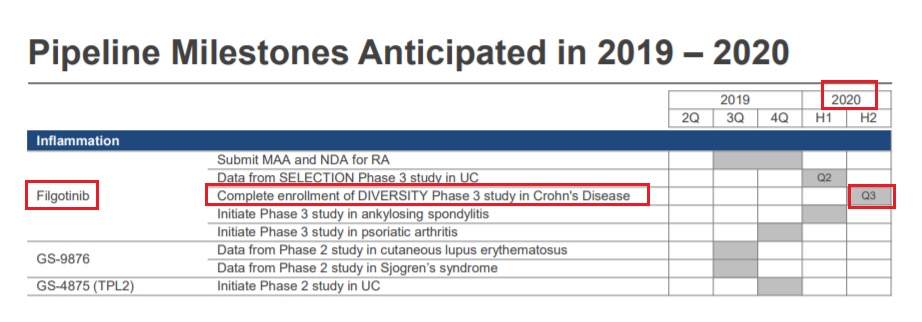
Remsima SC (infliximab biosimilar SC) / CelltrionEvaluating efficacy and safety of subcutaneous CT-P13 (CT-P13 SC) as maintenance therapy in patients with Crohn's disease (clinicaltrialsregister.eu) - Aug 1, 2019 - P3; N=600; Ongoing; Sponsor: Celltrion, Incfilgotinib (GLPG0634) / GileadFilgotinib clinical trial estimate: Data from P3 Diversity1 trial (NCT02914561) for Crohn’s disease in December 2019 (Oppenheimer) - Aug 1, 2019 - A subscription to Thomson ONE is required to gain full access to report 67660525; Page no: 2; REPORT TITLE: "Gilead Sciences Inc.: New CEO prepping for growth”; AUTHOR: Research Department; DATE: 07/22/2019TD-1473 / J&JTheravance Biopharma, Inc. reports second quarter 2019 financial results and provides business update (BioSpace) - Jul 31, 2019 - "Program Updates: TD-1473: Registrational Phase 2b/3 induction and maintenance study in ulcerative colitis (RHEA) and Phase 2 induction study in Crohn's disease (DIONE) progressing. Data from the Phase 2b portion of the ulcerative colitis and Phase 2 Crohn's disease studies planned late-2020."Xeljanz (tofacitinib) / PfizerPfizer reports second-quarter 2019 results (BioSpace) - Jul 29, 2019 - "Xeljanz globally, up 36% operationally, driven by: 103% operational growth in international markets...and 21% growth in the U.S., reflecting volume growth from the launches of the UC and psoriatic arthritis (PsA) indications as well as continued growth in the RA indication...partially offset primarily by lower revenues for: Enbrel internationally, down 16% operationally, primarily reflecting continued biosimilar competition in most developed Europe markets as well as the unfavorable impact of timing of government purchases in certain emerging markets..."Entyvio (vedolizumab) / TakedaEntyvio sales projection: Guidance of $4-5B peak (Wells Fargo) - Aug 3, 2019 - A subscription to Thomson ONE is required to gain full access to report 67660211; Page no: 1; REPORT TITLE: "Morphic Holding- Initiating coverage - MORF: Initiating at Outperform on oral integrin platform”; AUTHOR: Research Department; DATE: 07/22/2019filgotinib (GLPG0634) / GileadFilgotinib: Completion of enrollment in P3 DIVERSITY trial (NCT02914561) for Crohn's disease in Q3 2020 (Gilead) - Jul 31, 2019 - Q2 2019 Results